Tadalafil powder
Tadalafil powder is a medication used to treat erectile dysfunction (ED), benign prostatic hyperplasia (BPH), and pulmonary arterial hypertension.[1] It is a tablet taken by mouth.[1] Onset is typically within half an hour and the duration is up to 36 hours.[1] One of the largest manufacturer of tadalafil powder is CMOAPI which is a pharmaceutical manufacturer supplying top-quality tadalafil powder and tadalafil intermediate, and own the most comprehensive quality management system (ISO19001) and environmental management system (14001).
Tadalafil was approved for medical use in the United States in 2003.[1] It is available as a generic medication in the United States and United Kingdom.[2] In 2017, it was the 282nd most commonly prescribed medication in the United States, with more than one million prescriptions.[3][4]
Chemistry
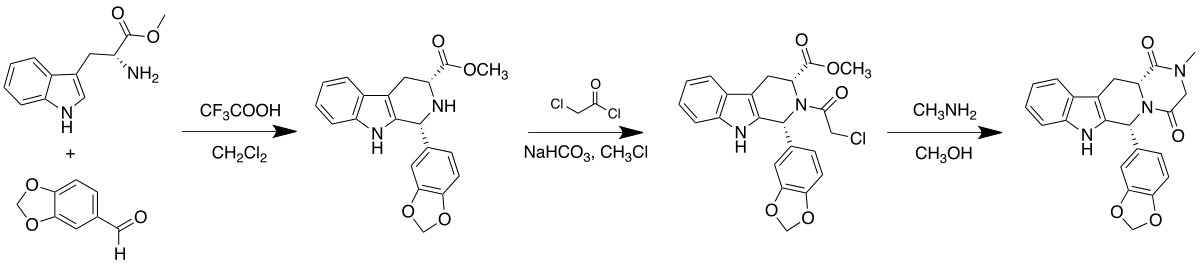
Tadalafil is an annulated 2,5-diketopiperazine.[5] It is also a 1,2,3,4-tetrahydro-β-carboline.
Tadalafil can be synthesized starting from (D)-tryptophan methyl ester and piperonal via a Pictet–Spengler reaction. This is followed by condensations with chloroacetyl chloride and methylamine to complete the diketopiperazine ring:[6]
Products
References
- ↑ 1.0 1.1 1.2 1.3 "Tadalafil Monograph for Professionals". American Society of Health-System Pharmacists. https://www.drugs.com/monograph/tadalafil.html.
- ↑ British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 796. ISBN 9780857113382.
- ↑ "The Top 300 of 2020". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Tadalafil - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Tadalafil.
- ↑ Borthwick AD (May 2012). "2,5-Diketopiperazines: Synthesis, Reactions, Medicinal Chemistry, and Bioactive Natural Products". Chemical Reviews 112 (7): 3641–3716. doi:10.1021/cr200398y. PMID 22575049.
- ↑ Baumann M (May 2011). "An overview of the key routes to the best selling 5-membered ring heterocyclic pharmaceuticals". Beilstein Journal of Organic Chemistry 7: 442–495. doi:10.3762/BJOC.7.57. PMC 3107522. PMID 21647262.